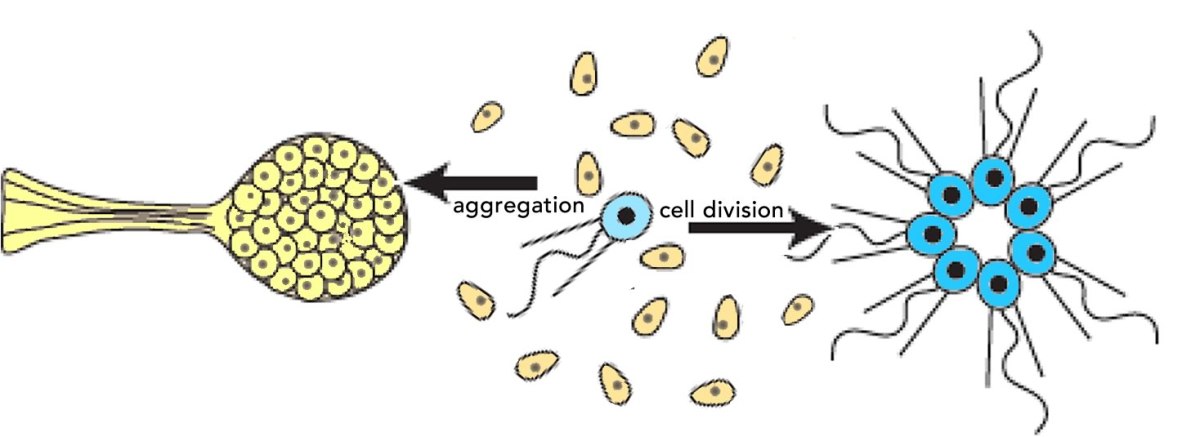
You might be constrained and contingent on past events, but you are not determined! (that said you are not exactly free either).
AI Generated Summary: Arguments for and against determinism and free will in relation to biological systems often overlook the fact that neither is entirely consistent with our understanding of how these systems function. The presence of stochastic, or seemingly random, events is widespread in biological systems and can have significant functional effects. These stochastic processes lead to a range of unpredictable but often useful behaviors. When combined with self-consciousness, such as in humans, these behaviors are not entirely determined but are still influenced by the molecular and cellular nature of living systems. They may feel like free actions, but they are constrained by the inherent biological processes.
Recently two new books have appeared arguing for (1) and against (2) determinism in the context of biological systems. There have also been many posts on the subject (here is the latest one by John Horgan. These works join an almost constant stream of largely unfounded, and bordering on anti-scientific speculation, including suggestions that consciousness has non-biological roots and exists outside of animals. Speaking as a simple molecular/cellular biologist with a more than passing interest in how to teach scientific thinking effectively, it seems necessary to anchor any meaningful discussion of determinism vs free will in clearly defined terms. Just to start, what does it mean to talk about a system as “determined” if we can not accurately predict its behavior? This brings us to a discussion of what are known as stochastic processes.
The term random is often use to describe noise, unpredictable variations in measurements or the behavior of a system. Common understanding of the term random implies that noise is without a discernible cause. But the underlying assumption of the sciences, I have been led to believe, is that the Universe is governed exclusively by natural processes; magical or supernatural processes are not necessary and are excluded from scientific explanations. The implication of this naturalistic assumption is that all events have a cause, although the cause(s) may be theoretically or practically unknowable. For example, there are the implications of Heisenberg’s uncertainty principle, which limits our ability to measure all aspects of a system. On the practical side, measuring the position and kinetic energy of each molecule (and the parts of larger molecules) in a biological system is likely to kill the cell. The apparent conclusion is that the measurement accuracy needed to consider a system, particularly a biological system as “determined” is impossible to achieve. In a strict sense, determinism is an illusion.
The question that remains is how to conceptualize the “random”and noisy aspects of systems. I would argue that the observable reality of stochasticity, particularly in biological systems at all levels of organization, from single cells to nervous systems, largely resolves the scientific paradox of randomness. Simply put, stochastic processes display a strange and counter-intuitive behavior: they are unpredictable at the level of individual events, but the behaviors of populations become increasingly predictable as population size increases. Perhaps the most widely known examples of stochastic processes are radioactive decay and Brownian motion. Given a large enough population of atoms, it is possible to accurately predict the time it takes for half of the atoms to decay. But knowing the half-life of an isotope does not enable us to predict when any particular atom will decay. In Schrödinger’s famous scenario a living cat is placed in an opaque box containing a radioactive atom; when the atom decays, it activates a process that leads to the death of the cat. At any particular time after the box is closed, it is impossible to predict with certainty whether the cat is alive or dead because radioactive decay is a stochastic process. Only by opening the box can we know for sure the state of the cat. We can, if we know the half-life of the isotope, estimate the probability that the cat is alive but rest assured, as a biologist who has a cat, at no time is the cat both alive and dead. We cannot know the “state of the cat” for sure until we open the box.
Something similar is going on with Brownian motion, the jiggling of pollen grains in water first described by Robert Brown in 1827. Einstein reasoned that “if tiny but visible particles were suspended in a liquid, the invisible atoms in the liquid would bombard the suspended particles and cause them to jiggle”. His conclusion was that Brownian motion provided evidence for the atomic and molecular nature of matter. Collisions with neighboring molecules provides the energy that drives diffusion; it drives the movement of molecules so that regulatory interactions can occur and provides the energy needed to disrupt such molecular interactions. The stronger the binding interaction between atoms or molecules the longer, ON AVERAGE, they will remain associated with one another. We can measure interaction affinities based on the half-life of interactions in a large enough population, but as with radioactive decay when exactly any particular complex dissociates cannot be predicted.
Molecular processes clearly “obey” rules. Energy is moved around through collisions, but we cannot predict when any particular event will occur. Gene expression is controlled by the assembly and disassembly of multicomponent complexes. The result is that we cannot know for sure how long a particular gene will be active or repressed. The result of such unpredictable assembly/disassembly events leads to what is known as transcriptional bursting; bursts of messenger RNA synthesis from a gene followed by periods of “silence” (3). A similar behavior is associated with the synthesis of polypeptides (4). Both processes can influence cellular and organismic behaviors. Many aspects of biological systems, including embryonic development, immune system regulation, and the organization and activity of neurons and supporting cells involved in behavioral responses to external and internal signals (5), display such noisy behaviors.
Why are biological systems so influenced by stochastic processes? Two simple reasons – they are composed of small, sometimes very small numbers of specific molecules. The obvious and universal extreme is that a cell typically contains one to two copies of each gene. Remember, a single change in a single gene can produce a lethal effect on the cell or organism that carries it. Whether a gene is “expressed” or not can alter, sometimes dramatically, cellular and system behaviors. The number of regulatory, structural, and catalytic molecules (typically proteins) present in a cell is often small leading to a situation in which the effects of large numbers do not apply. Consider a “simple” yeast cell. Using a range of techniques Ho et al (6) estimated that such cells contain about 42 million protein molecules. A yeast cell has around 5300 genes that encode protein components, with an average of 8400 copies of each protein. In the case of proteins present at low levels, the effects of noise can be functionally significant. While human cells are larger and contain more genes (~25,000) each gene remains at one to two copies per cell. In particular, the number of gene regulatory proteins tends to be on the low side. If you are curious the B10NUMB3R5 site hosted by Harvard University provides empirically derived estimates of the average number of various molecules in various organisms and cell types.
The result is that noisy behaviors in living systems are ubiquitous and their effects unavoidable. Uncontrolled they could lead to the death of the cell and organism. Given that each biological system appears to have an uninterrupted billion year long history going back to the “last universal common ancestor”, it is clear that highly effective feedback systems monitor and adjust the living state, enabling it to respond to molecular and cellular level noise as well as various internal and external inputs. This “internal model” of the living state is continuously updated to (mostly) constrain stochastic effects (7).
Organisms exploit stochastic noise in various ways. It can be used to produce multiple, and unpredictable behaviors from a single genome, and are one reason that identical twins are not perfectly identical (8). Unicellular organisms take advantage of stochastic processes to probe (ask questions of) their environment, and respond to opportunities and challenges. In a population of bacteria it is common to find that certain cells withdraw from active cell division, a stochastic decision that renders them resistant to antibiotics that kill rapidly dividing cells. These “persisters” are no different genetically from their antibiotic-sensitive relatives (9). Their presence enables the population to anticipate and survive environmental challenges. Another unicellular stochastically-regulated system is the bacteria E. coli‘s lac operon, a classic system that appears to have traumatized many a biology student. It enables the cell to ask “is there lactose in my environment?” How? A repressor molecule, LacI, is present in about 10 copies per cell. When more easily digestible sugars are absent the cell enters a stress state. In this state, when the LacI protein is knocked off the gene’s regulatory region there is a burst of gene expression. If lactose is present the proteins encoded by the operon are synthesized and enable lactose to enter and be broken down. One of the breakdown products inhibits the repressor protein, so that the operon remains active. No lactose present? The repressor rebinds and the gene goes silent (10). Such noisy regulatory processes enables cells to periodically check their environment so that genes stay on only when they are useful.
As noted by Honegger & de Bivort (11)(see also post on noise) decades of inbreeding with rodents in shared environments eliminated only 20–30% of the observed variance in a number of phenotypes. Such unpredictability can be beneficial. If an organism always “jumps” in the same direction on the approach of a predator it won’t take long before predators anticipate their behavior. Recent molecular techniques, particularly the ability to analyze the expression of genes at the single cell level, have revealed the noisiness of gene expression within cells of the same “type”. Surprisingly, in about 10% of human genes, only the maternal or the paternal version of a gene is expressed in a particular cell, leading to regions of the body with effectively different genomes. This process of “monoallelic expression” is distinct from the dosage compensation associated with the random “inactivation” of one or the other X-chromosomes in females. Monoallelic expression has been linked to “adaptive signaling processes, and genes linked to age-related diseases such as neurodegeneration and cancer” (12). The end result of noisy gene expression, mutation, and various “downstream” effects is that we are all mosaics, composed of clones of cells that behave differently due to noisy molecular differences.
Consider your brain. On top of the recently described identification of over 3000 neural cell types in the human brain (13), there is noisy as well as experience-dependent variation in gene expression, neuronal morphology and connectedness, and in the rates and patterns of neuronal firing due to differences in synaptic structure, position, strength, and other factors. Together these can be expected to influence how you (your brain) perceives and processes the external world, your own internal state, and the effects associated with the interaction between these two “models”. Of course the current state of your brain has been influenced, constrained by and contingent upon by past inputs and experiences, and the noisy events associated with its development. At the cellular level, the sum of these molecular and cellular interactions can be considered the consciousness of the cell, but this is a consciousness not necessarily aware of itself. In my admittedly naive view, as neural systems, brains, grow in complexity, they become aware of their own workings. As Godfrey-Smith (14) puts it, “brain processes are not causes of thoughts and experiences; they are thoughts and experiences”. Thoughts become inputs into the brain’s model of itself.
What seems plausible is that as nervous systems increase in complexity, processing increasing amounts of information including information arising from its internal workings, it may come to produce a meta-model that for reasons “known” only to itself needs to make sense of those experiences, feelings, and thoughts. In contrast to the simpler questions asked by bacteria, such as “is there an antibiotic or lactose in my world?”, more complex (neural) systems may ask “who is to blame for the pain and suffering in the world?” I absent-mindedly respond with a smile to a person at a coffeehouse, and then my model reconsiders (updates) itself depending, in part, upon their response, previous commitments or chores, and whether other thoughts distract or attract “me”. Out of this ferment of updating models emerges self-conscious biological activities – I turn to chat or bury my head back in my book. How I (my model) responds is a complex function of how my model works and how it interprets what is going on, a process influenced by noise, genetics, and past experiences; my history of rewards, traumas, and various emotional and “meaningful” events.
Am I (my model) free to behave independently from these effects? no! But am I (my model) determined by them, again no! The effects of biological noise in its various forms, together with past and present events will be reinforced or suppressed by my internal network and my history of familial, personal, and social experiences. I feel “free” in that there are available choices, because I am both these models and the process of testing and updating them. Tentative models of what is going on (thinking fast) are then updated based on new information or self-reflection (thinking slower). I attempt to discern what is “real” and what seems like an appropriate response. When the system (me) is working non-pathologically, it avoids counter-productive, self-destructive ideations and actions; it can produce sublime metaphysical abstractions and self-sacrificing (altruistic) behaviors. Mostly it acts to maintain itself and adapt, often resorting to and relying upon the stories it tells itself. I am neither determined nor free, just an organism coping, or attempting to cope, with the noisy nature of existence, its own internal systems, and an excessively complex neural network.
Added notes: Today (5 Dec. 23) was surprised to discover this article (Might There Be No Quantum Gravity After All?) with the following quote “not all theories need be reversible, they can also be stochastic. In a stochastic theory, the initial state of a physical system evolves according to an equation, but one can only know probabilistically which states might occur in the future—there is no unique state that one can predict.” Makes you think! Also realized that I should have cited Zechner et al (added to REF 11) and now I have to read “Free will without consciousness? by Mudrik et al., 2022. Trends in Cog. Sciences 26: 555-566.
Literature cited
- Sapolsky, R.M. 2023. Determined: A Science of Life Without Free Will. Penguin LLC US
- Mitchell, K.J. 2023. Free Agents: How Evolution Gave Us Free Will. Princeton.
- Fukaya, T. (2023). Enhancer dynamics: Unraveling the mechanism of transcriptional bursting. Science Advances, 9(31), eadj3366.
- Livingston, N. M., Kwon, J., Valera, O., Saba, J.A., Sinha, N.K., Reddy, P., Nelson, B. Wolfe, C., Ha, T.,Green, R., Liu, J., & Bin Wu (2023). Bursting translation on single mRNAs in live cells. Molecular Cell.
- Harrison, L. M., David, O., & Friston, K. J. (2005). Stochastic models of neuronal dynamics. Philosophical Transactions of the Royal Society B: Biological Sciences, 360(1457), 1075-1091.
- Ho, B., Baryshnikova, A., & Brown, G. W. (2018). Unification of protein abundance datasets yields a quantitative Saccharomyces cerevisiae proteome. Cell systems, 6, 192-205.
- McNamee & Wolpert (2019). Internal models in biological control. Annual review of control, robotics, and autonomous systems, 2, 339-364.
- Czyz, W., Morahan, J. M., Ebers, G. C., & Ramagopalan, S. V. (2012). Genetic, environmental and stochastic factors in monozygotic twin discordance with a focus on epigenetic differences. BMC medicine, 10, 1-12.
- Manuse, S., Shan, Y., Canas-Duarte, S.J., Bakshi, S., Sun, W.S., Mori, H., Paulsson, J. and Lewis, K., 2021. Bacterial persisters are a stochastically formed subpopulation of low-energy cells. PLoS biology, 19, p.e3001194.
- Vilar, J. M., Guet, C. C. and Leibler, S. (2003). Modeling network dynamics: the lac operon, a case study. J Cell Biol 161, 471-476.
- Honegger & de Bivort. 2017. Stochasticity, individuality and behavior & Zechner, C., Nerli, E., & Norden, C. 2020. Stochasticity and determinism in cell fate decisions. Development, 147, dev181495.
- Cepelewicz 2022. Nature Versus Nurture? Add ‘Noise’ to the Debate.
- Johansen, N., Somasundaram, S.,Travaglini, K.J., Yanny, A.M., Shumyatcher, M., Casper, T., Cobbs, C., Dee, N., Ellenbogen, R., Ferreira, M., Goldy, J., Guzman, J., Gwinn, R., Hirschstein, D., Jorstad, N.L.,Keene, C.D., Ko, A., Levi, B.P., Ojemann, J.G., Nadiy, T.P., Shapovalova, N., Silbergeld, D., Sulc, J., Torkelson, A., Tung, H., Smith, K.,Lein, E.S., Bakken, T.E., Hodge, R.D., & Miller, J.A (2023). Interindividual variation in human cortical cell type abundance and expression. Science, 382, eadf2359.
- Godfrey-Smith, P. (2020). Metazoa: Animal life and the birth of the mind. Farrar, Straus and Giroux.